Article: Understanding the Global Health Problem of Antimicrobial Resistance and How to Solve It
Infectious diseases for which current medical treatments are not effective are among the leading cause of death of people of all ages globally. These are caused by microbial pathogens (principally bacteria) that have emerged under the evolutionary selective pressure imposed by overuse and misuse of antibiotics to show antimicrobial resistance (AMR). Recently published data show an alarming rise in AMR. In 2019, drug-resistant infections directly killed 1.27 million people, more than HIV/AIDS (864,000) or malaria (643,000) (Murray et al., 2022). The ability of bacteria to resist a drug to which they have historically shown sensitivity is in part due to the generation of biofilm.
A biofilm is formed when bacteria attach to a surface, living or inert, and create a physical barrier that is very difficult to penetrate. It is a formidable weapon to fight not only antibacterial treatments but also an infected individual’s immune system. Therefore, in-depth understanding of the mechanisms by which biofilm develops can contribute to driving different approaches to overcome AMR. Staphylococcus aureus, particularly methicillin-resistant S. aureus (MRSA), is a big problem in hospitals, because it gets inadvertently attached to medical devices like catheters and saline drips and is extremely hard to clean off. As a result, a long-stay patient, who is likely to have their health already compromised, can easily be infected. For this reason, MRSA has become the focus of a considerable research effort into AMR and how it can be overcome.
In their recent journal article entitled “Biofilm Formation by Pathogenic Bacteria: Applying a Staphylococcus aureus Model to Appraise Potential Targets for Therapeutic Intervention”, Professor Andrew Taylor-Robinson from VinUniversity and his collaborator Dr Zahra Sedarat use S. aureus biofilm as an example to consider cutting-edge research to identify and evaluate molecular targets to combat AMR.
A lot of work has been done to understand the characteristics of biofilms, how they develop and how they are resistant to various antimicrobial agents. To produce effective therapies against biofilm, we need to understand its four stages of development: attachment, multiplication, maturation, and dispersal. This is because what treatment to choose and how effective it is greatly depend on the stage biofilm is in. For example, an ideal time to attack biofilm is when it is less stable in its multiplication stage.
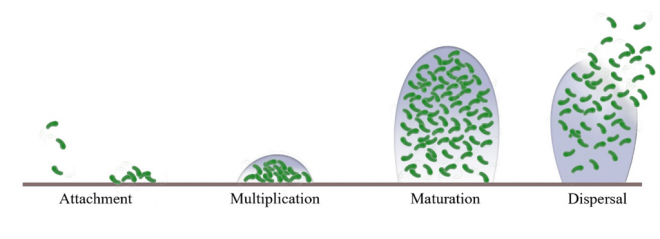
Figure 1. Schematic representation of Staphylococcus aureus biofilm development
The most common anti-biofilm treatment is antibiotics. Used to specifically treat bacteria, when encountering AMR, often the strategy is to raise the dosage of an antibiotic or to combine with other antimicrobial agents. However, this approach does not always work as bacteria can use several different mechanisms to resist. Therefore, as Dr. Taylor-Robinson said when interviewed, there is a pressing need for new classes of antibiotics that will help to resolve the current situation. But as much as anything, it requires to actually slow the pace of development of AMR, which means less reliance on and less inappropriate demand for the current range of antibiotics. This also requires designing and promoting the use of novel alternative anti-biofilm treatments.
Other anti-biofilm treatments that are reviewed in the article include vaccines, antibodies, biofilm-degrading enzymes, probiotics, rhamnolipids, photodynamic therapy, phytochemicals, nanoparticles, bacteriophages, antimicrobial peptides, chelators and sulfhydryl compounds, and laser therapy. All these agents have their own strengths and weaknesses, so caution is required when considering which would be the preferred treatment for a patient.
In conclusion, the wealth of research on MRSA provides valuable insights on principles that can be applied to other biofilm-forming pathogenic bacteria. Echoing the article’s message, in his interview, Dr Taylor-Robinson mentioned the urgent need for innovative approaches to biofilm prevention and treatment. He also stressed that reducing the escalating global health burden of AMR calls for improved public awareness of appropriate use of antibiotics and coordinated international efforts to enhance antimicrobial stewardship.
Dr Taylor-Robinson is also a principal investigator of an exciting initiative on wastewater epidemiological surveillance that is predicated on the methodology underpinning an early detection system for COVID-19. This project is a long-term collaboration between the Smart Health Center at VinUniversity and the University of Illinois Urbana-Champaign. It is developing a non-invasive, privacy-preserved tool to monitor the spread of antibiotic resistance genes throughout a community. It has the potential to provide an early warning system for future infectious disease outbreaks. Its findings will help the interdisciplinary research team to unravel the evolution of AMR in response to human behavior, community practices, and medical interventions.
To read the full article: click here.
Andrew Taylor-Robinson is Professor of Microbiology; Immunology in the College of Health Sciences, VinUniversity.
_____________________________________
References
Antimicrobial Resistance Collaborators (2022). Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet, 399(10325), 629-655. https://doi.org/10.1016/S0140-6736(21)02724-0
Sedarat Z. & Taylor-Robinson A.W. (2022). Biofilm Formation by Pathogenic Bacteria: Applying a Staphylococcus aureus Model to Appraise Potential Targets for Therapeutic Intervention. Pathogens, 11(4), 388. https://doi.org/10.3390/pathogens11040388



![[Job Opening]: Teaching Assistant for Research Minicourse Development at VinUniversity](https://vinuni.edu.vn/research/wp-content/uploads/2024/07/hiring-7062502_1280-300x143.jpg)